Diffusion and Exchange
Detection of tumor response to treatment in the clinic is largely based on imaging a reduction of tumor size as a marker of treatment response. However, reductions in tumor size may take several weeks and are therefore difficult to detect if a rapid evaluation of a treatment strategy is needed. Therefore, for early detection of tumor response to treatment, novel response markers are to be found. Alternative response markers, e.g. 18F-FDG-uptake, which is reduced in gastrointestinal tumors already 24 h after treatment with Imatinib, have been demonstrated but involve ionizing radiation and thus make repeated exams difficult. Further alternative techniques, such as diffusion-weighted imaging (DWI) have been shown to report on a reduction in tumor cellularity resulting in increased ADCs as a marker for treatment response that precedes reduction in tumor size. Importantly, the MRI contrast being generated using the diffusion properties of water molecules does not involve exogenous contrast agents. It therefore represents an exquisite non-invasive way to probe the tumor microenvironment using commercially available clinical MRI equipment which renders clinical translation of diffusion-based MRI protocols feasible.
In our group we optimize and develop protocols for probing diffusion microstructure in vivo using DWI for applications in oncology. ADCs and image performance are evaluated using established phantoms for ADC validation and characterisation of image artifacts. The quality of in vivo diffusion-weighted MRI acquisition heavily depends on the reduction of motion artifacts which we want to tackle both by respiratory gating and retrospective artefact reduction.
However, ADC does not always increase with successful treatment progression and is a relatively late marker of cell death only sensitive upon excessive necrosis. Therefore, there is a need for novel IBs to image early necrotic cell death.
Recently, a novel MRI contrast mechanism based on pulsed field gradient magnetic resonance methods has been introduced for in vivo measurements, which detects transmembrane water exchange, quantified by the so-called apparent exchange rate (AXR). The technique named ‘filter-exchange imaging’ (FEXI) relies on a dual diffusion-weighted MRI sequence and has been pioneered by a group of researchers from Lund University. In our recent publication in Nature Biotechnology we showed that it is even sensitive enough to pick up a small amount of ca. 100,000 cells that have been genetically modified to express water channel proteins thereby altering their transmembrane water permeability (Figure 1).
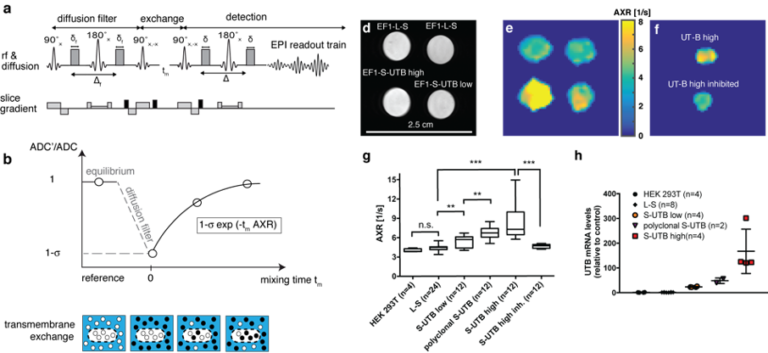
Figure 1. Imaging transmembrane water-exchange. a, The filter-exchange imaging (FEXI) sequence consists of a diffusion filter module, a storage/exchange module, and signal detection using an EPI-readout. b, At equilibrium, with no diffusion filter, a reference ADC is acquired. When the diffusion filter is applied, signal from fast diffusing extracellular water is suppressed, indicated by the black dots, resulting in a different weighting of the signal contributions from the two compartments and leading to a reduction in the measured ADC’. This decrease in ADC is determined by the filter efficiency . With increasing mixing times, tm, between the filter and detection modules, exchange of water between the two compartments results in an increase in the measured ADC. Relaxation back to the equilibrium ADC can be described by the apparent exchange rate constant, AXR. d, Reference proton image of tubes containing dense pellets of the indicated cell lines. e, AXR maps of the cell pellets shown in d). f, AXR map of pellets of EF1-S-UTB high cells. For one of the pellets the cells had been incubated with 170 mM N-N’-Dimethylthiourea. g, Independent experiments show increased AXR with increasing UT-B expression (n = 4-24) and a return to base-level AXR after inhibition of UT-B. h, Real-time PCR measurements of UT-B mRNA expression levels. Data shown in g and h are mean ± SD. Significance was tested using a Mann-Whitney-Wilcoxon test; significance levels: n.s. p > 0.05, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001. Figure adapted from our recent publication in Nature Biotechnology
Cell death plays a key role in cancer staging and treatment strategy. Various mechanisms of cell death in cancer are known, including apoptosis, necrosis and necroptosis. The integrity of the cell membrane is often compromised after the onset of cell death. In yeast cells, we have demonstrated that AXR is sensitive in changes to membrane permeabilization that cannot be detected by changes in ADC (see Figure 2, Kaika et al. 2024).
In addition, preclinical data in acute myeloid leukemia (AML) and subcutaneous EL4 lymphoma cells show that transmembrane water exchange rate is an early biomarker of cell death when quantified by apparent exchange rate (AXR) calculated by filter exchange spectroscopy (FEXSY) or imaging (FEXI).
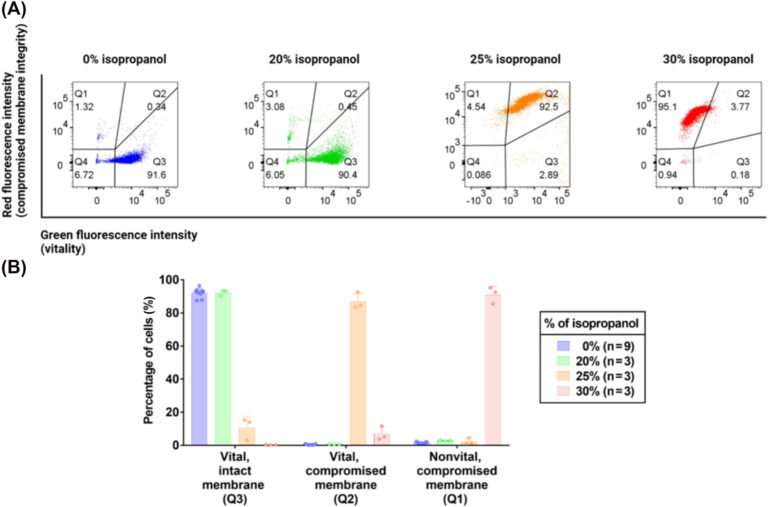
Figure 2. Analysis of isopropanol-treated yeast cells by flow cytometry. (a) Representative dual-color fluorescence scatterplots from flow cytometry. (b) Barplot of the percentage of vital/intact membrane (Q3), vital/compromised membrane (Q2), and nonvital/compromised membrane (Q1) cells that were detected by flow cytometry in the yeast suspension treated with 20%, 25%, and 30% isopropanol. 0% isopropanol corresponds to the untreated CFDA, AM, and PI-stained sample, which served as a control sample for the flow cytometry analysis of treated samples. This control sample was prepared following the same conditions as the treated sample, except replacing the isopropanol with water. In both the (a) and (b) plots, blue, green, orange, and red correspond to the entire populations of the cell suspensions that were treated with 0%, 20%, 25%, and 30% isopropanol, respectively. CFDA, AM, 5-carboxyfluorescein diacetate, acetoxymethyl ester; PI, propidium iodide.
Relevant publications:
- Athanasia Kaika, Geoffrey J Topping, Luca Nagel, Franz Schilling; ’Filter-exchange spectroscopy is sensitive to gradual membrane degradation’, NMR in Biomedicine (2024); e5202; doi: 10.1002/nbm.5202
- Mathias Schillmaier*, Athanasia Kaika*, Geoffrey J Topping, Rickmer Braren, Franz Schilling; ‘Repeatability and reproducibility of apparent exchange rate measurements in yeast cell phantoms using filter-exchange imaging’, Magnetic Resonance Materials in Physics, Biology and Medicine (2023); doi: 10.1007/s10334-023-01107-w
- Mathias Schillmaier, Athanasia Kaika, Franz Schilling; ‚Disentangling intercompartment exchange from restricted diffusion‘, in: ‚Advanced Diffusion Encoding Methods in MRI‘, Royal Society of Chemistry (2020); 24:154
- Franz Schilling, Susana Ros, De-En Hu, Paula D’Santos, Sarah McGuire, Elizabeth Mannion, Andre A. Neves, Kevin M. Brindle; ‘The urea transporter – a novel substrate-free MRI gene reporter detected using transmembrane water exchange imaging’, Nature Biotechnology (2017); 35:75-80
- Benedikt Feuerecker, Markus Durst, Michael Michalik, Günter Schneider, Dieter Saur, Marion Menzel, Markus Schwaiger, Franz Schilling; ´Hyperpolarized 13C Diffusion MRS of Co-Polarized Pyruvate and Fumarate to Measure Lactate Export and Necrosis´; Journal of Cancer (2017); 8(15):3078
- Franz Schilling, Stephan Düwel, Ulrich Köllisch, Markus Durst, Rolf F. Schulte, Steffen J. Glaser, Axel Haase, Angela M. Otto, Marion I. Menzel; ‘Diffusion of hyperpolarized 13C metabolites in tumor cell spheroids using real-time NMR spectroscopy’, NMR in Biomedicine (2013); 26(5):557-568
Latest News
-
 ISMRM 2025 HonoluluMay 16, 2025/0 Comments
ISMRM 2025 HonoluluMay 16, 2025/0 Comments -
-

-
 Welcome SilvesterMay 1, 2025/
Welcome SilvesterMay 1, 2025/ -
 20th European Molecular Imaging Meeting BilbaoMarch 14, 2025/
20th European Molecular Imaging Meeting BilbaoMarch 14, 2025/ -
 Welcome OscarMarch 1, 2025/
Welcome OscarMarch 1, 2025/ -
 International Quantum ForumFebruary 7, 2025/
International Quantum ForumFebruary 7, 2025/ -
 HyperMet LaunchFebruary 2, 2025/
HyperMet LaunchFebruary 2, 2025/ -
 Happy holidaysDecember 20, 2024/
Happy holidaysDecember 20, 2024/ -
 Dr. rer. nat. Martin GrasheiDecember 19, 2024/
Dr. rer. nat. Martin GrasheiDecember 19, 2024/
