Multimodal Metabolic Imaging
Magnetic resonance spectroscopic imaging (MRSI) has been used to study biochemical processes non-invasively, although it suffers from a low sensitivity and requires long scan times. The invention of hyperpolarization (HP) techniques has shifted these limits, allowing signal enhancements of 13C-labelled metabolites by more than five orders of magnitude. However, hyperpolarized signals decay with the spin-lattice relaxation time (T1) which is on the order of a few tens of seconds, limiting the time available for signal acquisition and requiring fast signal acquisition.
Hyperpolarized [1-13C]pyruvate is most often used in vivo, because of its key role in glucose metabolism, its fast metabolic conversion to lactate, its high achievable polarization level and a relatively long T1. Tumors readily internalize and metabolize pyruvate to lactate by lactate dehydrogenase (LDH-A). The expression of LDH-A is upregulated in many tumors, which results in an increased pyruvate-to-lactate conversion that can be quantified with MRSI.
We attempt to assess the flux of glucose through the glycolytic pathway simultaneously with both FDG-PET and pyruvate imaging. In a recent study, we established and validated a workflow at a clinical whole-body PET/MR for the multiparametric characterization of the glycolytic flux in a pre-clinical MAT-B-III breast cancer model. We analyzed the in vivo correlation of FDG uptake with pyruvate-to-lactate conversion and performed a longitudinal study with a group of tumor-bearing rats to study the effect of changing tumor cellularity on metabolic PET and MRSI data (see Figure 1).
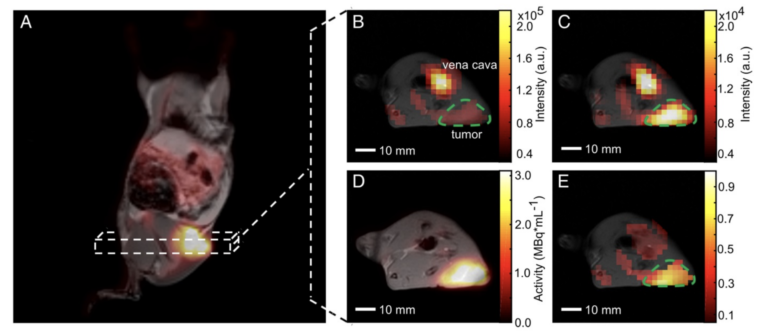
Figure 1. Qualitative comparison of simultaneously imaged FDG uptake and pyruvate-to-lactate exchange in MAT-B-III tumor-bearing rats. (A) Coronal slice of a late frame PET image overlaid on the respective slices of an MPRAGE image. Static axial images of pyruvate (B), lactate (C) and FDG (D) in the same orientation. (E) is the ratio of (C) and (B) showing a higher lactate to pyruvate ratio in the tumor than observed in the surrounding tissue, which correlates well with the high FDG uptake shown in (D). Note that for CSI images, only pixels with a signal > 20% of the maximum signal of hyperpolarized pyruvate and lactate are displayed, respectively. Note further that the pyruvate signal is about a factor of ten higher than that of lactate and that the nominal resolution of 13C-images was two-fold higher than the one of images for quantitative analysis.
In this study, single-frequency bSSFP (without multi-echoes) was used for consecutively imaging individual hyperpolarized metabolites in vitro and in vivo. The Warburg effect was imaged by observing the exchange of [1-13C]pyruvate and [1-13C]lactate. In a recent study, we investigated multi-echo-bSSFP-IDEAL at 3T. Compared to previous results, the spatial and temporal resolution was substantially improved such that observation of the metabolic conversion in one slice or 3D metabolite mapping became possible (see Figure 2).
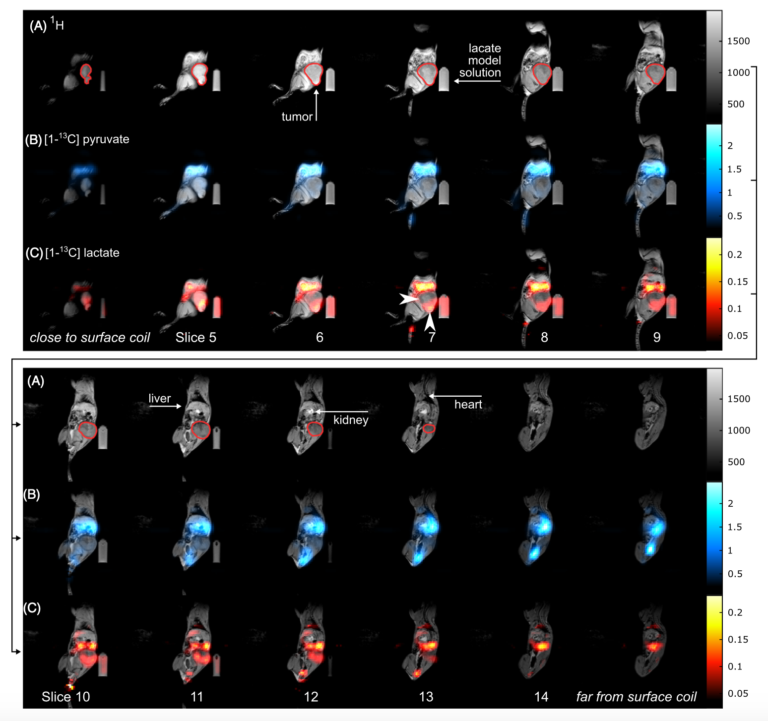
Figure 2: Sagittal slices of a 3D metabolite map. In vivo 1H MRI (A, gray) and 3D 13C metabolite maps of pyruvate (B, blue) and lactate (C, red) of a tumor bearing rat obtained with a 3D me-bSSFP. The scan was started right after injection was finished and ~45 s after dissolution. The injection lasted 26 s and the 3D scan time was 6.8 s. Strong signal of lactate and pyruvate was observed in the stomach, the blood vessels, liver, kidneys and heart (in descending order of signal intensity). The subcutaneous tumor (green outline) exhibited strong and spatially variable lactate signal, which may be a sign of metabolic heterogeneity. Note, that the image color scales start at 2.5% and 10% of the maximum intensity for pyruvate and lactate, respectively. Not all slices of the 3D volume are shown.
In addition, a new approach for imaging hyperpolarized nuclei – “off resonant, spectrally selective 3D balanced steady-state free precession (bSSFP)” has been developed in my research group and has already been demonstrated in healthy mice (see Figure 3, Skinner et al. 2023). The sequence uses broad, off- resonant radiofrequency (RF) excitation pulses that, with appropriate choice of flip angle, allow selective excitation of pyruvate and lactate. These metabolites play a central role in energy metabolism. Dynamic in vivo images were acquired in healthy mice at a resolution of (1.75 mm)3, one of the highest resolutions to date for dynamic 3D imaging of hyperpolarized 13C molecules. The broadband RF excitations used within the sequence require only a short RF pulse duration, allowing short repetition times (TR) to be achieved. Current efforts focus on the translation of pulse sequences for hyperpolarized imaging to the clinic on a 3T human scanner.
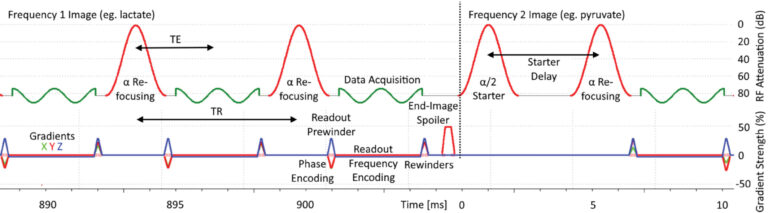
Figure 3: RF and gradient waveform timing diagram for the 3D bSSFP sequence. It shows annotations for various RF (top) and gradient (bottom) pulses and RF signal acquisition windows on an excerpt from the ParaVision sequence simulation output showing several TR across a transition between metabolite images. Slice selection and refocusing gradients are not used during starter and refocusing pulses. RF phase is fixed constant for all RF pulses. An α/2 flip angle preparation pulse precedes the train of 192 α flip angle refocusing pulses (although different flip angles are not represented in this diagram). For metabolic imaging, the RF pulses alternate between two central frequencies that target two distinct metabolite chemical shifts, here separated by the dotted line at time 0, after each single-metabolite full-volume acquisition. The refocusing RF pulse nominal flip angles (90° for lactate, 4° for pyruvate) also vary between the two metabolites.
Hyperpolarized 13C MRI provides a unique insight into metabolism and tissue function. It utilizes molecular transformations as a mechanism for imaging contrast. The most successful hyperpolarized 13C MRI probe so far is [1-13C]pyruvate. Pyruvate is a key metabolite in energy metabolism and, as the end product of glycolysis, it is further metabolized to lactate, alanine or to acetyl-CoA (after cleavage of 13CO2), which then enters the tricarboxylic acid (TCA) cycle. By quantifying pyruvate’s downstream metabolism, glycolytic tissue can be differentiated from tissue dominated by aerobic respiration. Besides dissolution dynamic nuclear polarization (dDNP), which operates at liquid helium temperature close to absolute zero temperature, other methods for generating hyperpolarized 13C-labeled probes are based on polarization transfer from parahydrogen and require substantially less technological efforts and cost. In a recent collaborative effort, we have demonstrated preclinical evidence for safe and reliable polarization and in vivo administration of [1-13C]pyruvate using parahydrogen (See Figure 4, Nagel et al. 2023). We aim to further translate this approach to the clinic with first experiments in human cancer patients planned in 2026 at the TUM University Hospital.
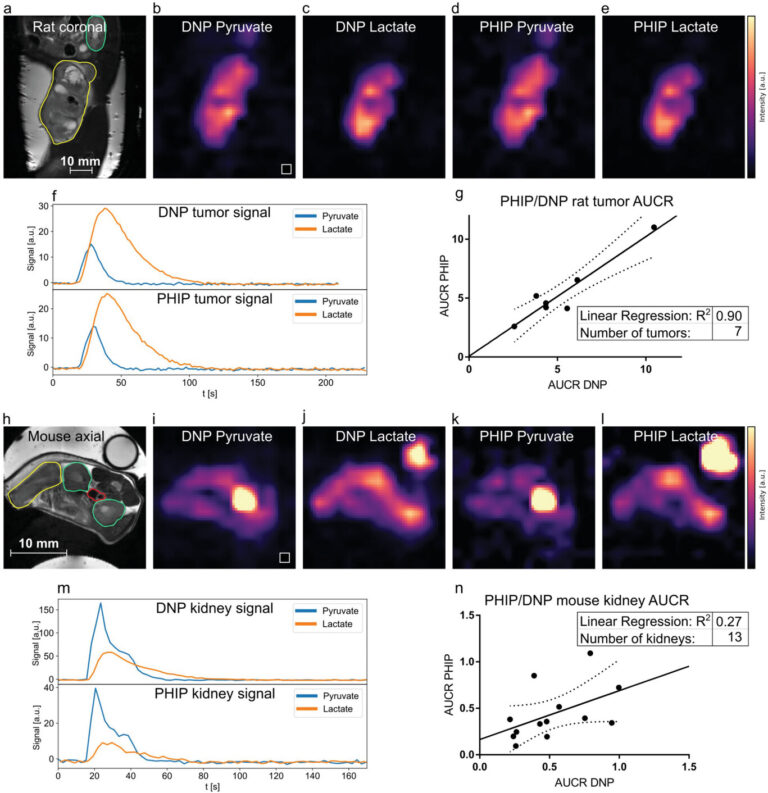
Figure 4: Comparison of metabolism in rat tumors and mice kidneys between PHIP and d-DNP hyperpolarized [1-13C]pyruvate using a dual metabolite targeted spectrally-selective bSSFP sequence. a–f), PHIP and d-DNP datasets of a Mat B III tumor-bearing rat showing the distribution of injected hyperpolarized pyruvate b,d) and metabolized lactate c,e). In the corresponding anatomical reference (a), the tumor and kidney are drawn in yellow and green, respectively. The tumor 3D ROI time curves of pyruvate and lactate are shown in (f). The signal intensities b,c,d,e,i,j,k,l) were scaled to maximum value for better comparability. g), comparison of rat tumor AUCR of PHIP and d-DNP showing very good correlation between the two methods (R2 = 0.90). h–l), pyruvate and lactate distributions in a tumor-bearing mouse are shown, with the corresponding anatomical reference in (h). Tumor (yellow), kidneys (green), and the blood vessel (red) ROIs are depicted. A [1-13C]lactate enriched phantom for RF power calibration is visible in the top right in the panels (h,j,l). m), signal intensity time curves from the left kidney (green ROI). n), correlation of PHIP and d-DNP AUCRs of mouse kidneys (R2 = 0.27). Dashed lines in (g, n) indicate the 95% confidence interval.
Relevant publications:
- Pascal Wodtke, Martin Grashei, Franz Schilling; ‘Quo Vadis Hyperpolarized 13C MRI’, Zeitschrift für Medizinische Physik – Journal of Medical Physics (2024); doi: 10.1016/j.zemedi.2023.10.004
- Luca Nagel, Martin Gierse, Wolfgang Gottwald, Zumrud Ahmadova, Martin Grashei, Pascal Wolff, Felix Josten, Senay Karaali, Christoph A Müller, Sebastian Lucas, Jochen Scheuer, Christoph Müller, John Blanchard, Geoffrey J Topping, Andre Wendlinger, Nadine Setzer, Sandra Sühnel, Jonas Handwerker, Christophoros Vassiliou, Frits HA van Heijster, Stephan Knecht, Michael Keim§, Franz Schilling§, Ilai Schwartz§; ‘Parahydrogen-Polarized [1-13C] Pyruvate For Reliable and Fast Preclinical Metabolic Magnetic Resonance Imaging’, Advanced Science (2023); doi: 10.1002/advs.202303441 (cover page article for issue 30/2023)
- Jason G. Skinner*, Geoffrey J. Topping*, Luca Nagel, Irina Heid, Christian Hundshammer, Martin Grashei, Frits H.A. van Heijster, Rickmer Braren, Franz Schilling; ‘Spectrally selective bSSFP using off-resonant RF excitations permits high spatiotemporal resolution 3D metabolic imaging of hyperpolarized [1-13C]Pyruvate-to-[1-13C]lactate conversion’, Magnetic Resonance in Medicine (2023); 1-16. doi: 10.1002/mrm.29676 (MRM editor´s pick)
- Henri de Maissin, Philipp Groß, Obaid Mohiuddin, Moritz Weigt, Luca Nagel, Marvin Herzog, Zirun Wang, Robert Willing, Wilfried Reichardt, Martin Pichotka, Lisa Heß, Thomas Reinheckel, Henning Jessen, Robert Zeiser, Michael Bock, Dominik von Elverfeldt, Maxim Zaitsev, Sergey Korchak, Stefan Glöggler, Jan-Bernd Hövener, Eduard Chekmenev, Franz Schilling, Stephan Knecht, Andreas Benjamin Schmid; ‘In Vivo Metabolic Imaging of [1-13C] Pyruvate-d3 Hyperpolarized By Reversible Exchange With Parahydrogen’, Angewandte Chemie (2023); doi: 10.1002/anie.202306654
- Martin Gierse*, Luca Nagel*, Michael Keim, Sebastian Lucas, Tobias Speidel, Tobias Lobmeyer, Gordon Winter, Felix Josten, Senay Karaali, Maximilian Fellermann, Jochen Scheuer, Christoph Müller, Frits van Heijster, Jason Skinner, Jessica Löffler, Anna Parker, Jonas Handwerker, Alastair Marshall, Alon Salhov, Bilal El-Kassem, Christophoros Vassiliou, John W. Blanchard, Román Picazo-Frutos, James Eills, Holger Barth, Fedor Jelezko, Volker Rasche, Franz Schilling, Ilai Schwartz§, Stephan Knecht§; ‘Parahydrogen-Polarized Fumarate for Preclinical in Vivo Metabolic Magnetic Resonance Imaging’, Journal of the American Chemical Society (2023); 145(10):5960-5969
- Geoffrey J. Topping, Irina Heid, Marija Trajkovic-Arsic, Lukas Kritzner, Martin Grashei, Christian Hundshammer, Maximilian Aigner, Jason G. Skinner, Rickmer Braren, Franz Schilling; ‘Hyperpolarized 13C Spectroscopy with Simple Slice-and-Frequency-Selective Excitation’, Biomedicines (2021); 9(2):121
- Elisabeth Bliemsrieder, Georgios Kaissis, Martin Grashei, Geoffrey J. Topping, Jennifer Altomonte, Christian Hundshammer, Fabian Lohöfer, Irina Heid, Dominik Keim, Selamawit Gebrekidan, Marija Trajkovic-Arsic, Aline Winkelkotte, Katja Steiger, Roman Nawroth, Jens Siveke, Markus Schwaiger, Marcus Makowski, Franz Schilling, Rickmer Braren, ‘Hyperpolarized 13 C pyruvate magnetic resonance spectroscopy for in vivo metabolic phenotyping of rat HCC’, Scientific reports (2021); 11 (1):1–9
- Christoph A. Müller, Christian Hundshammer, Miriam Braeuer, Jason G. Skinner, Stephan Berner, Jochen Leupold, Stephan Düwel, Stephan G. Nekolla, Sven Månsson, Adam E Hansen, Dominik von Elverfeldt, Jan H. Ardenkjaer‐Larsen, Franz Schilling, Markus Schwaiger, Jürgen Hennig, Jan‐Bernd Hövener; ‘Dynamic 2D and 3D mapping of hyperpolarized pyruvate to lactate conversion in vivo with efficient multi‐echo balanced steady‐state free precession at 3 T’, NMR in Biomedicine (2020); 33(6):e4291
- Geoffrey J. Topping, Christian Hundshammer, Luca Nagel, Martin Grashei, Maximilian Aigner, Jason G. Skinner, Rolf F. Schulte, Franz Schilling; ‘Acquisition strategies for spatially resolved magnetic resonance detection of hyperpolarized nuclei’, Magnetic Resonance Materials in Physics, Biology and Medicine (2020); 33:221-256
- Christian Hundshammer, Miriam Braeuer, Christoph Müller, Adam Hansen, Mathias Schillmaier, Stephan Düwel, Benedikt Feuerecker, Steffen Glaser, Axel Haase, Wilko Weichert, Katja Steiger, Jorge Cabello, Franz Schilling, Jan-Bernd Hövener, Andreas Kjaer, Stephan Nekolla, Markus Schwaiger; ‘Simultaneous characterization of tumor cellularity and the Warburg effect with PET, MRI and hyperpolarized 13C-MRSI’, Theranostics (2018); 8(17):4765
Latest News
-
 Happy holidaysDecember 20, 2024/0 Comments
Happy holidaysDecember 20, 2024/0 Comments -
 Dr. rer. nat. Martin GrasheiDecember 19, 2024/
Dr. rer. nat. Martin GrasheiDecember 19, 2024/ -
 QuE-MRT Annual Meeting in MunichOctober 26, 2024/
QuE-MRT Annual Meeting in MunichOctober 26, 2024/ -
 TUM Supervisory Award MH 2024October 25, 2024/
TUM Supervisory Award MH 2024October 25, 2024/ -
 Athanasia Kaika, PhDOctober 18, 2024/
Athanasia Kaika, PhDOctober 18, 2024/ -
 Kevin Brindle new TUM Hans Fischer Senior FellowOctober 1, 2024/
Kevin Brindle new TUM Hans Fischer Senior FellowOctober 1, 2024/ -
 New DFG Research Unit “HyperMet”September 26, 2024/
New DFG Research Unit “HyperMet”September 26, 2024/ -
 Kevin Brindle’s Retirement SymposiumSeptember 19, 2024/
Kevin Brindle’s Retirement SymposiumSeptember 19, 2024/ -
 Gorter Award 2nd Place for Martin GrasheiSeptember 6, 2024/
Gorter Award 2nd Place for Martin GrasheiSeptember 6, 2024/ -
 DACH Conference in TübingenSeptember 4, 2024/
DACH Conference in TübingenSeptember 4, 2024/
