pH Biosensors
Many efforts have been undertaken to develop an imaging method to characterize hypoxic and acidic regions within tumors, namely a quantitative non-invasive pH imaging technique. However, so far, no technique to non-invasively image extracellular pH has been applicable routinely in the clinic. In humans, pH is mainly regulated by the carbon dioxide/bicarbonate buffer within a narrow pH range, in the blood between pH 7.35 and 7.45. Locally, deviations from the systemic pH are often caused by pathologies, such as cancer, inflammation, infection, ischemia, renal failure or pulmonary disease. Our team identified the pH-sensitive molecule [1,5-13C2]zymonic acid (ZA) which we recently reported in a publication in Nature Communications (Figure 1).
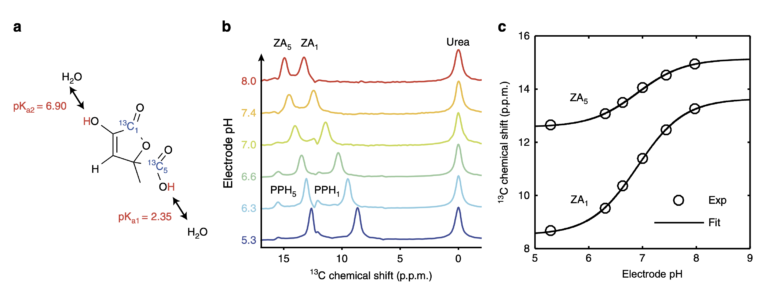
Figure 1. Chemical structure, mechanism and calibration of the pH dependency of ZA at 7 T. a, ZA bears two exchangeable protons, one in a carboxy group with an acid dissociation constant pKa1 = 2.35 and a second one in an enolic hydroxy group with pKa2 = 6.90. b, Spectra of hyperpolarized ZA and urea in buffer phantoms at different pH values. The pH sensitivity in the physiological range is due to pKa2 and results in a change of the chemical shifts of the two 13C-labeled carbon positions with respect to the pH-insensitive 13C-urea peak as a function of pH. Additional peaks of a decay product of ZA, parapyruvate hydrate (PPH), can be identified. c, The fit of the data from (b) results in a calibration curve with a direct dependence of pH on the chemical shift.
To increase the sensitivity in 13C experiments, we developed a hyperpolarization protocol for ZA. Results in vivo in kidneys and tumors (Figure 2) clearly demonstrate that ZA represents a feasible novel candidate for pH imaging with advantages over existing methods. The demonstrated robustness of using ZA for extracellular pH imaging, its lack of toxicity and its accuracy render this new pH imaging technique promising for further development.
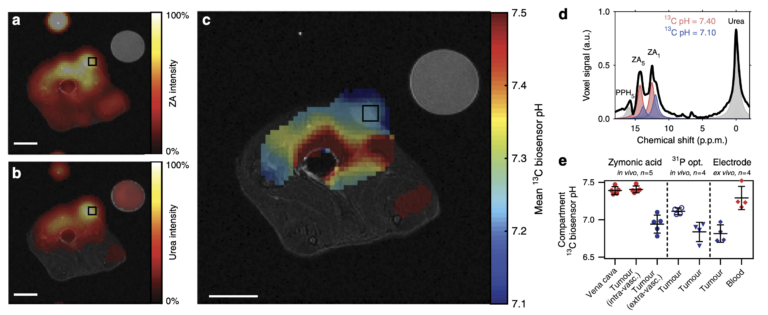
Figure 2: Hyperpolarized ZA in vivo pH measurements show an acidic tumor pH at 7 T. Representative tumor data from a hyperpolarized 13C measurement (colored) in an axial slice overlayed on anatomical proton images (grayscale). A calibration phantom containing 13C urea and the catheter used for injection are visible. Both (a) ZA and (b) urea accumulate in the MAT B III tumor. (c) The mean pH map shows a lower pH value in the tumor compared to the surrounding tissue. For all 13C images, intensity windows are based on sufficiently high signal levels for either (intensity images) or both (pH images) ZA and urea. Spectra from tumor voxels (d), shown for one representative animal) are best fitted with two pairs of ZA peaks (red, blue) and consistently show different pH values in the tumor in comparison to the vena cava, demonstrated in five animals (e), individual datapoints and mean ± s.d.) and compared with three other interstitial/extracellular pH measurements in four animals, namely 31P MRS in vivo using 3-APP, a needle-type optical sensor in vivo and an ex vivo tissue electrode pH measurement. Scale bars, 1 cm.
In another recent study, we show that hyperpolarized and deuterated [1,5-13C2, 3,6,6,6-D4]zymonic acid (ZAd) can be used as an even more sensitive in vivo biosensor to non-invasively image pH. Furthermore, we demonstrate real-time imaging of dynamic pH changes in vitro.
Also, alternative molecules for pH quantification, such as Z-OMPD, are being investigated within my group. We introduced Z-OMPD in 2023 as a new molecular sensor for hyperpolarized 13C MRI (Grashei et al. 2023). The good pH sensitivity and hyperpolarization properties combined with tailored MRI protocols allow simultaneous imaging of pH, renal perfusion and filtration in the sub minute range (Figure 3). Increases in sensitivity of our pH imaging approach are expected from selective 13C-labelling and deuteration. We currently evaluate our novel pH imaging technique in different existing tumor models following different treatment strategies, such as immunotherapies, radioligand therapies, or chemotherapies. We will take a translational path starting from rodent tumor models to feline patients with spontaneous neoplastic diseases measured on a clinical scanner. A thorough analysis of the methodological advances and results of the in vivo studies will be used to evaluate the potential of in vivo pH mapping for clinical translation.
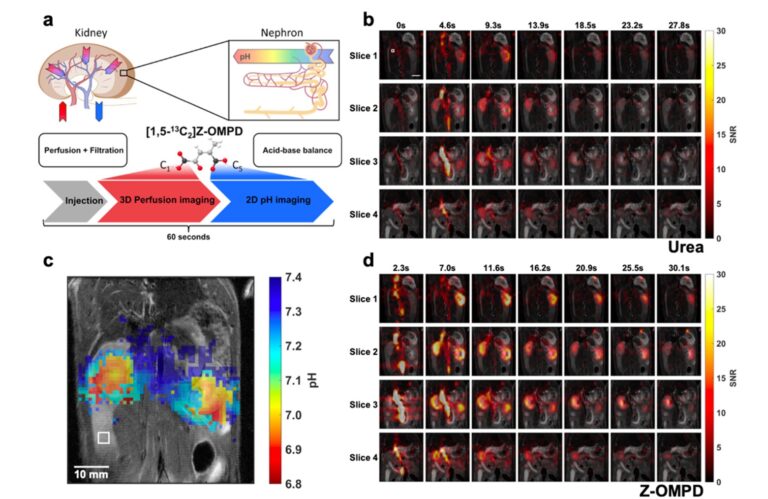
Figure 3: Simultaneous in vivo imaging of renal perfusion, filtration, and acid-base balance. a: Scheme for combined imaging of renal kidney perfusion and filtration in 3D and renal pH in 2D. b: Image time series of co-injected hyperpolarized [13C]urea. c: Average pH maps with high contrast between renal cortex and pelvis d: Image time series of the C1-magnetization of hyperpolarized Z- OMPD. The higher SNR compared to [13C]urea together with the high spatial resolution allows better assessment of first-pass perfusion and renal filtration from the cortex (renal periphery) to the pelvis (renal center) when injected at identical concentrations. Hyperpolarized acquisitions are overlaid on an anatomical T2-weighted image. From our publication (Grashei et al. 2023).
Relevant publications:
- Martin Grashei*, Pascal Wodtke*, Jason G Skinner, Sandra Sühnel, Nadine Setzer, Thomas Metzler, Sebastian Gulde, Mihyun Park, Daniela Witt, Hermine Mohr, Christian Hundshammer, Nicole Strittmatter, Natalia S Pellegata, Katja Steiger, Franz Schilling; ‘Simultaneous magnetic resonance imaging of pH, perfusion and renal filtration using hyperpolarized 13C-labelled Z-OMPD’, Nature Communications (2023); 14:5060; doi: 10.1038/s41467-023-40747-3
- Wai-Yan Ryana Fok*, Martin Grashei*, Jason G. Skinner, Björn H. Menze, Franz Schilling; ‘Prediction of multiple pH compartments by deep learning in magnetic resonance spectroscopy with hyperpolarized 13C-labelled zymonic acid’, EJNMMI Research (2022); 12,24
- Martin Grashei, Christian Hundshammer, Frits H.A. van Heijster, Geoffrey J. Toppping, Franz Schilling; ‘pH Dependence of T2 for Hyperpolarizable 13C-Labelled Small Molecules Enables Spatially Resolved pH Measurement by Magnetic Resonance Imaging’, Pharmaceuticals (2021); 14 (4):327 (cover page)
- Christian Hundshammer, Martin Grashei, Alexandra Greiner, Steffen J. Glaser, Franz Schilling; ‘pH Dependence of T1 for 13C‐Labelled Small Molecules Commonly Used for Hyperpolarized Magnetic Resonance Imaging’, ChemPhysChem (2019); 20:798-802
- Simone Köcher, Stephan Düwel, Christian Hundshammer, Steffen J. Glaser, Franz Schilling, Josef Granwehr, Christoph Scheurer; ‘Ab Initio Simulation of pH-Sensitive Biomarkers in Magnetic Resonance Imaging’, The Journal of Physical Chemistry A (2018); 122(40):7983-7990
- Christian Hundshammer, Stephan Düwel, David Ruseckas, Geoffrey Topping, Piotr Dzien, Christoph Müller, Benedikt Feuerecker, Jan-Bernd Hövener, Axel Haase, Markus Schwaiger, Steffen J. Glaser, Franz Schilling; ‘Hyperpolarized Amino Acid Derivatives as Multivalent Magnetic Resonance pH Sensor Molecules’, Sensors (2018); 18(2):600
- Stephan Düwel, Christian Hundshammer, Malte Gersch, Benedikt Feuerecker, Katja Steiger, Achim Buck, Axel Walch, Axel Haase, Steffen J. Glaser, Markus Schwaiger and Franz Schilling; ‘Imaging of pH in vivo using hyperpolarized 13C-labeled zymonic acid’, Nature Communications (2017); 8:15126
- Christian Hundshammer, Stephan Düwel, Simone Köcher, Malte Gersch, Benedikt Feuerecker, Christoph Scheurer, Axel Haase, Steffen J. Glaser, Markus Schwaiger, Franz Schilling; ´Deuteration of hyperpolarized 13C-labelled zymonic acid enables sensitivity-enhanced dynamic MRI of pH´; ChemPhysChem (2017); 18(18):2422-2425 (cover page)
- Christian Hundshammer, Stephan Düwel, Franz Schilling; ´Imaging of Extracellular pH Using Hyperpolarized Molecules´, Israel Journal of Chemistry (2017); 57(9)788-799
Latest News
-
 ISMRM 2025 HonoluluMay 16, 2025/0 Comments
ISMRM 2025 HonoluluMay 16, 2025/0 Comments -
-

-
 Welcome SilvesterMay 1, 2025/
Welcome SilvesterMay 1, 2025/ -
 20th European Molecular Imaging Meeting BilbaoMarch 14, 2025/
20th European Molecular Imaging Meeting BilbaoMarch 14, 2025/ -
 Welcome OscarMarch 1, 2025/
Welcome OscarMarch 1, 2025/ -
 International Quantum ForumFebruary 7, 2025/
International Quantum ForumFebruary 7, 2025/ -
 HyperMet LaunchFebruary 2, 2025/
HyperMet LaunchFebruary 2, 2025/ -
 Happy holidaysDecember 20, 2024/
Happy holidaysDecember 20, 2024/ -
 Dr. rer. nat. Martin GrasheiDecember 19, 2024/
Dr. rer. nat. Martin GrasheiDecember 19, 2024/
